One of the most persistent assumptions in healthcare is that patients are reluctant—or even resistant—to clinical trials. But when you ask patients directly, a different story emerges.
At Responsum Health, we regularly ask our community members what they want to learn about, what they worry about, and where they feel ready to engage. Across all of our condition-specific communities and channels, one theme continues to stand out: patients are far more open to clinical trial information than many expect.
What Our Data Shows
Over the past year, we asked community members a simple but important question:
“Are you interested in learning about clinical trial opportunities?”
Here’s how 3,881 respondents across all Responsum communities answered:
- Yes, very interested: 66.3%
- Maybe / open to learning more: 13.2%
- Not right now: 20.5%
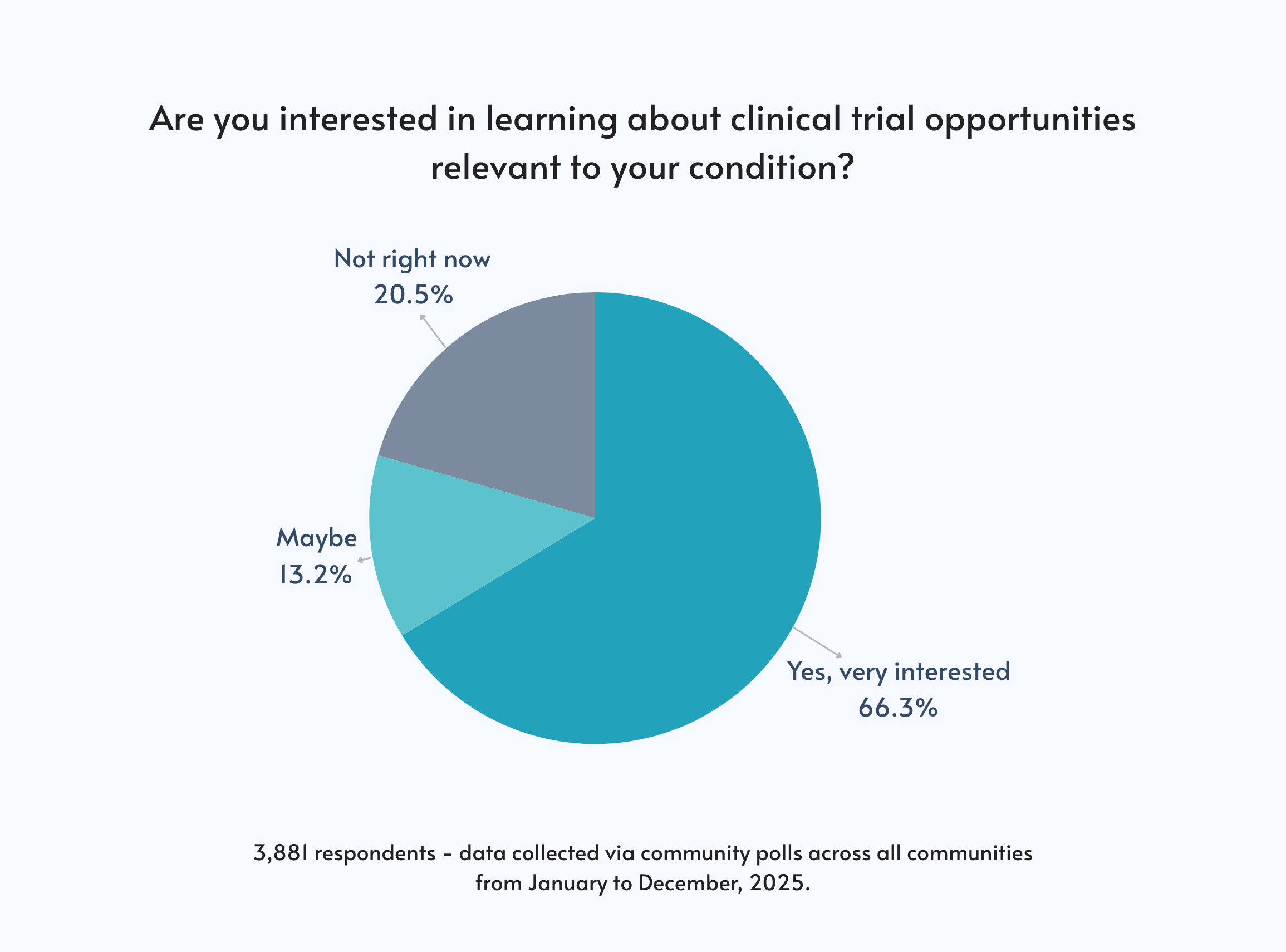
In other words, nearly 80% of respondents are at least open to learning about clinical trials. That’s not hesitation—that’s curiosity and potential readiness.
Interest Doesn’t Mean Pressure—It Means Opportunity
It’s important to clarify what this data does not mean. Patients aren’t asking to be rushed into studies or treated like leads. What they’re telling us is that they want clear, trustworthy, and relevant information—delivered in a way that respects where they are in their health journey.
For many patients, “interest” looks like:
- Wanting to understand what trials are available
- Learning how safety and eligibility work
- Knowing whether participation could be relevant now or in the future
- Feeling empowered to ask questions—without obligation
This is exactly where education and community play a critical role.
Why This Matters for Research and Industry Partners
Recent research shows that patient hesitancy—not lack of interest—is a major barrier to clinical trial enrollment. A study published in ScienceDirect found that 65% of patients feel hesitant about participating, mainly due to concerns about safety, placebos, trust in pharmaceutical companies, and logistical challenges. The study also showed that patients with lower health literacy are significantly more hesitant.
Importantly, hesitancy is not permanent. About 40% of patients said they would be more likely to participate if a trusted healthcare provider clearly explained the trial and recommended it. This highlights a critical opportunity for partners: delivering education and trust-building early can meaningfully improve patient readiness.
How Community Changes the Conversation
The same study makes clear that patient hesitancy is shaped by fear, mistrust, and lack of understanding, challenges that can’t be solved at the point of recruitment alone.
Community-based engagement helps address these barriers upstream. In Responsum communities, patients build health literacy over time, learn about research in plain language, and gain confidence through ongoing education and peer support. This prepares them to have more informed, productive conversations with their providers when trial opportunities arise.
Rather than replacing traditional recruitment, community strengthens it, creating a more informed, confident, and research-ready patient population.
Final Thoughts
The takeaway is simple but powerful: patients are not disengaged from research—they’re waiting to be approached the right way.
With over 66% of our community members expressing strong interest in learning about clinical trials, and many more open to the conversation, the opportunity is clear. The challenge, and responsibility, is to meet that interest with education, transparency, and respect.
If you’re a partner looking to improve how patients learn about and access clinical research, we’d love to talk. Together, we can build pathways that empower patients while advancing meaningful, ethical research.
👉 Contact Responsum Health to explore how our communities can support patient-first clinical trial education and access.
Sources:
- Responsum Health Community Polls
- Journal of the National Medical Association via Science Direct. “Overcoming Patient Hesitancy in Clinical Trial Enrollment” sciencedirect.com


